
 The most exotic use of argon is in the tyre of luxury cars to protect the rubber and reduce road noise. Argon is used in incandescent light bulbs to stop oxygen from corroding the filament. Argon is also used in technical SCUBA diving to inflate the drysuit due to its nonreactive, heat isolating effect. Argon is used in radio tubes and Geiger counters. Argon is used in some types of arc welding such as gas metal arc welding and gas tungsten arc welding as well as processes that require shielding from other atmospheric gases. On earth the vast majority of argon is the isotope argon-40, which arises from the radioactive decay of potassium-40. Argon is produced commercially by fractional distillation of liquefied air with (for high purity argon) catalytic burning of left over traces of oxygen. Argon is obtained from the air as a byproduct of the production of oxygen and nitrogen. Argon has a face-centered cubic crystal structure. The Van der waals radius of argon is 188 pm. The first, second and third Ionization energies of argon is 1520.6 kJ/mol, 2665.8 kJ/mol and 3931 kJ/mol respectively.
The most exotic use of argon is in the tyre of luxury cars to protect the rubber and reduce road noise. Argon is used in incandescent light bulbs to stop oxygen from corroding the filament. Argon is also used in technical SCUBA diving to inflate the drysuit due to its nonreactive, heat isolating effect. Argon is used in radio tubes and Geiger counters. Argon is used in some types of arc welding such as gas metal arc welding and gas tungsten arc welding as well as processes that require shielding from other atmospheric gases. On earth the vast majority of argon is the isotope argon-40, which arises from the radioactive decay of potassium-40. Argon is produced commercially by fractional distillation of liquefied air with (for high purity argon) catalytic burning of left over traces of oxygen. Argon is obtained from the air as a byproduct of the production of oxygen and nitrogen. Argon has a face-centered cubic crystal structure. The Van der waals radius of argon is 188 pm. The first, second and third Ionization energies of argon is 1520.6 kJ/mol, 2665.8 kJ/mol and 3931 kJ/mol respectively. 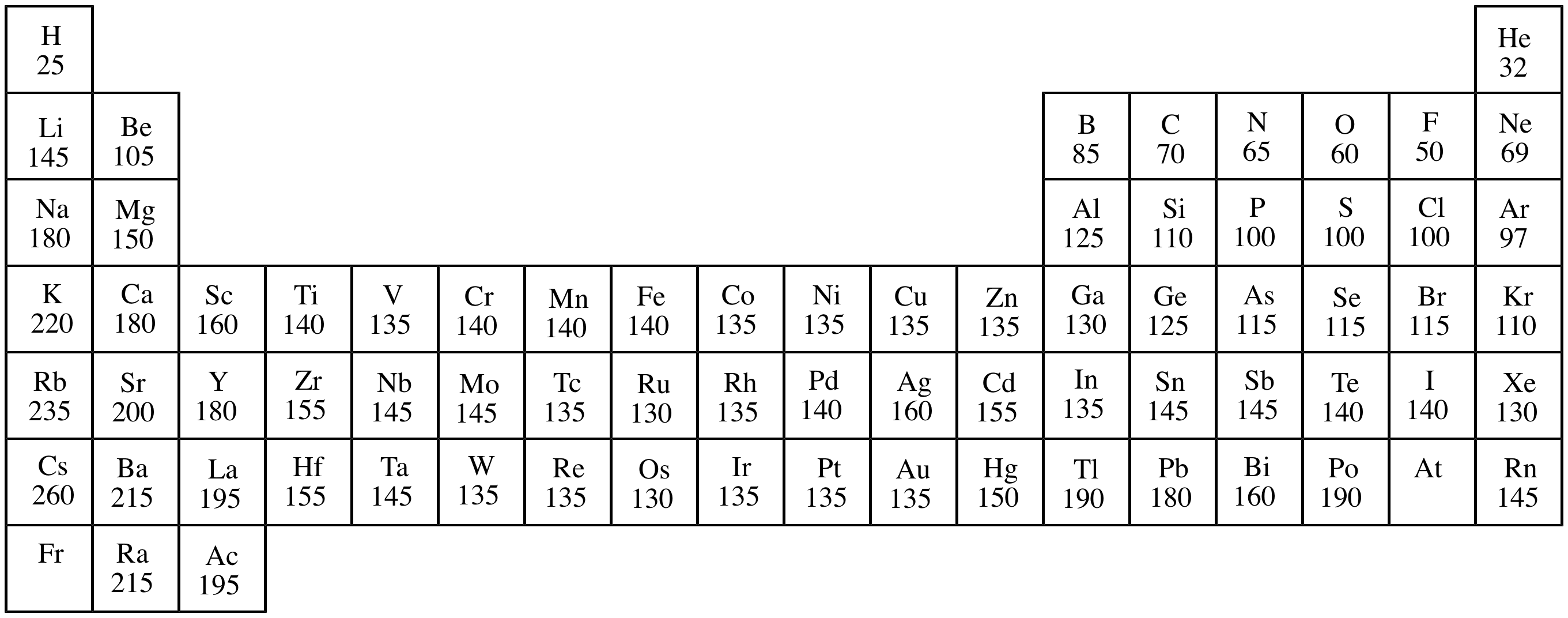 The electronegativity of argon is unknown. Atomic weight is the average mass of the atom. Argon atoms do not combine with one another nor have they been observed to combine chemically with atoms of any other element. Argon is diamagnetic at standard pressure and temperature. Thermal conductivity of Argon is 17.72 x 10 -3 W/(m.k). The electrical resistivity of argon is very low. According to Royal Society of Chemistry (RSC) Argon makes up 0.94 percent of Earth’s atmosphere and is the third most abundant atmospheric gas. Argon has a Molar heat capacity of 20.85 kJ/mol. The heat of vaporization of argon is 6.53 kJ/mol. The heat of fusion of argon is 1.18 kJ/mol. The critical point of argon 150.687K, 4.863 Mpa. The triple point of argon is 83.8058K, 68.89 kPa. Density of argon at standard temperature and pressure is 0.0017837 grams per cubic centimeter (1.78g/L). Argon gas condenses to a colorless liquid at -185.8 oC (-302.4 oF) and to a crystalline solid at -189.4 oF (-308.9 oF). Argon is a gas at standard temperature and pressure. Argon is chemically inert under most conditions and forms no confirmed stable compounds at room temperature. Argon has approximately the same solubility as oxygen and it is 2.5 times as soluble in water as nitrogen. Argon is a colorless, odorless gas that is totally inert to other substances. The outermost (valence) shell of argon has eight electrons, making it exceedingly stable and thus chemically inert. Electrons per shell in Argon is 2,8,8. Argon has an electron configuration of (Ne)3s 23p 6. Other elements in the same category include: helium, neon, argon, krypton, xenon and radon.
The electronegativity of argon is unknown. Atomic weight is the average mass of the atom. Argon atoms do not combine with one another nor have they been observed to combine chemically with atoms of any other element. Argon is diamagnetic at standard pressure and temperature. Thermal conductivity of Argon is 17.72 x 10 -3 W/(m.k). The electrical resistivity of argon is very low. According to Royal Society of Chemistry (RSC) Argon makes up 0.94 percent of Earth’s atmosphere and is the third most abundant atmospheric gas. Argon has a Molar heat capacity of 20.85 kJ/mol. The heat of vaporization of argon is 6.53 kJ/mol. The heat of fusion of argon is 1.18 kJ/mol. The critical point of argon 150.687K, 4.863 Mpa. The triple point of argon is 83.8058K, 68.89 kPa. Density of argon at standard temperature and pressure is 0.0017837 grams per cubic centimeter (1.78g/L). Argon gas condenses to a colorless liquid at -185.8 oC (-302.4 oF) and to a crystalline solid at -189.4 oF (-308.9 oF). Argon is a gas at standard temperature and pressure. Argon is chemically inert under most conditions and forms no confirmed stable compounds at room temperature. Argon has approximately the same solubility as oxygen and it is 2.5 times as soluble in water as nitrogen. Argon is a colorless, odorless gas that is totally inert to other substances. The outermost (valence) shell of argon has eight electrons, making it exceedingly stable and thus chemically inert. Electrons per shell in Argon is 2,8,8. Argon has an electron configuration of (Ne)3s 23p 6. Other elements in the same category include: helium, neon, argon, krypton, xenon and radon. 
Cobalt is categorized as a noble gas in the periodic table.In the periodic table, Argon is located in group 18 (noble gases), period 3, p-block.Argon is obtained from the air as a byproduct of the production of oxygen and nitrogen. On Earth, the vast majority of argon is the isotope argon-40 which arises from the radioactive decay of potassium-40. Occurrence In NatureĪrgon makes up 0.93% of the earth’s atmosphere, making it the third most abundant atmospheric gas. They were then left with a gas that would not react and when they examined its spectrum they saw new groups of red and green lines, confirming that it was a new element. They removed all the nitrogen from the gas they had extracted from air and did this by reaching it with hot magnesium, forming the solid magnesium nitride. Sir William Ramsay and Lord Rayleigh collaborated in isolating Argon from air. The examination entailed a carefully designed sequence of experiments guided by an analysis of the resulting observation. It was isolated by examination of the residue obtained by removing nitrogen, oxygen, carbon dioxide and water from clean air. Argon was discovered as a result of trying to explain why the density of nitrogen extracted from air differed from that obtained by the decomposition of ammonia. What You Need To Know About Argon DiscoveryĪrgon was discovered by an English chemist Lord Rayleigh and a Scottish chemist Sir William Ramsay in 1894 at University College London.







 0 kommentar(er)
0 kommentar(er)
